
How many valence electrons are in an atom of Phosphorus MakeTheBrainHappy
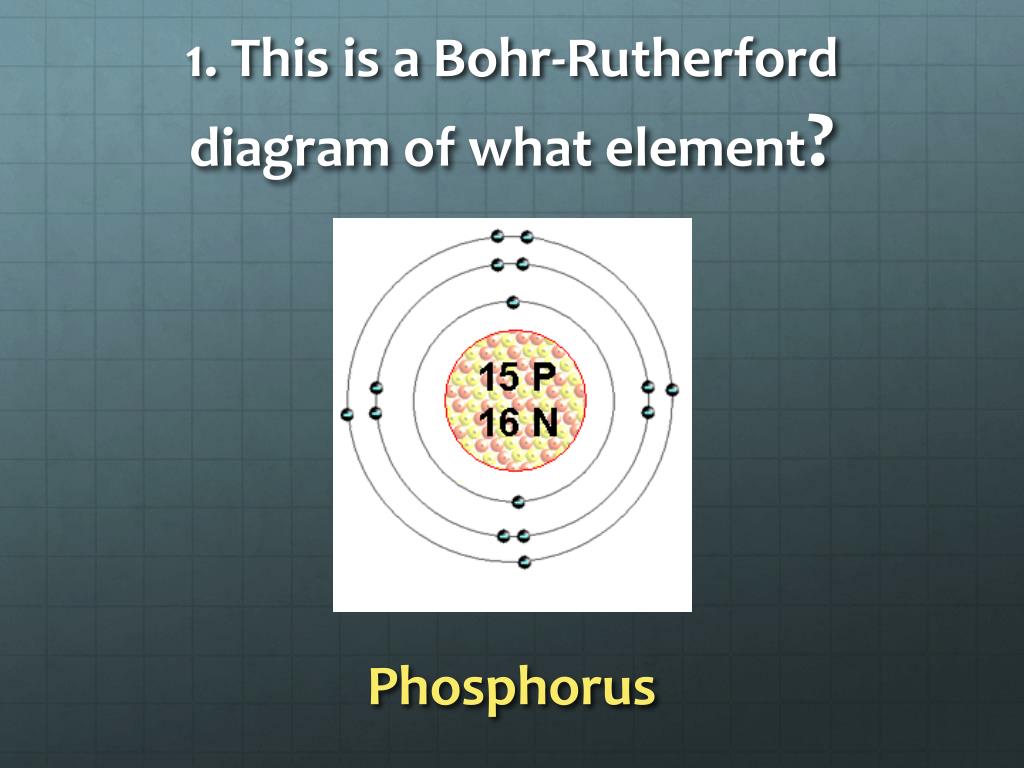
4) The following diagram is a Bohr-Rutherford diagram of one element from the periodic table: To which group and period does this element belong? A) Period 3 group 4. B) Period 4 group 4. C) Period 3 group 1. D) Period 1 group 3. 5) Referring to the periodic table, find an element that has the same number of electron shells and
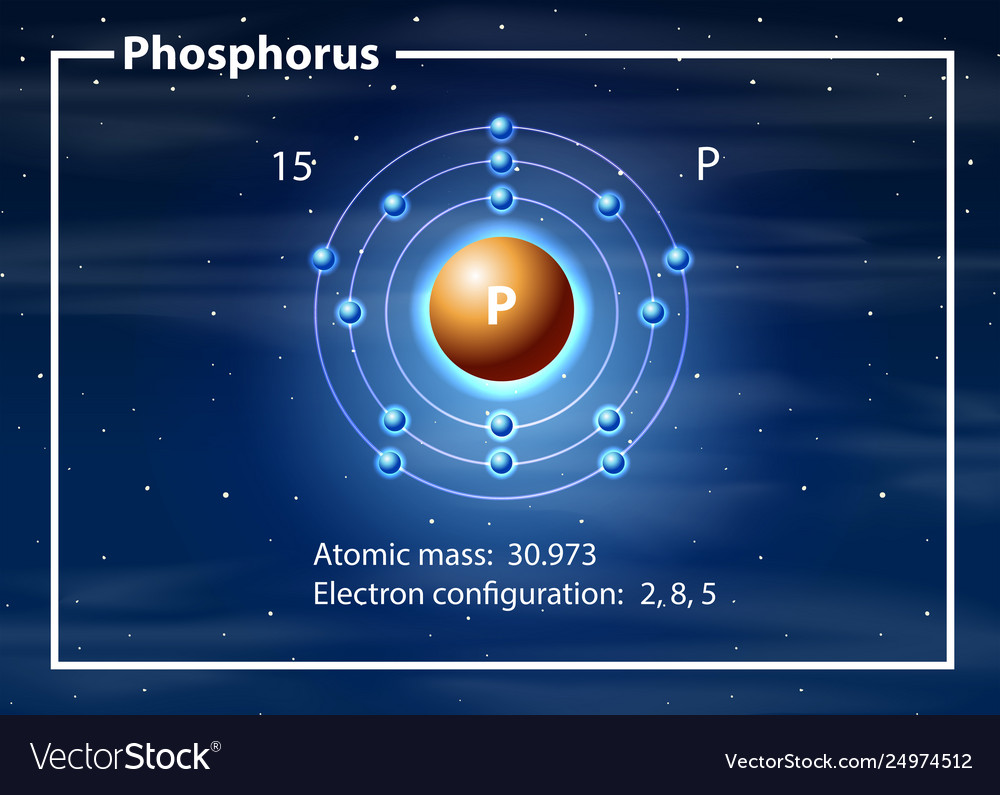
Phosphorus Bohr Diagram
Find step-by-step Biology solutions and your answer to the following textbook question: (a) Draw the Bohr-Rutherford diagram (without neutrons) for an atom of each of the following elements: lithium, oxygen, calcium; and phosphorus. (b) Draw the; Bohr-Rutherford diagram (without neutrons) for the ion formed by each of the elements in (a). (c) Write the chemical symbol for each ion.
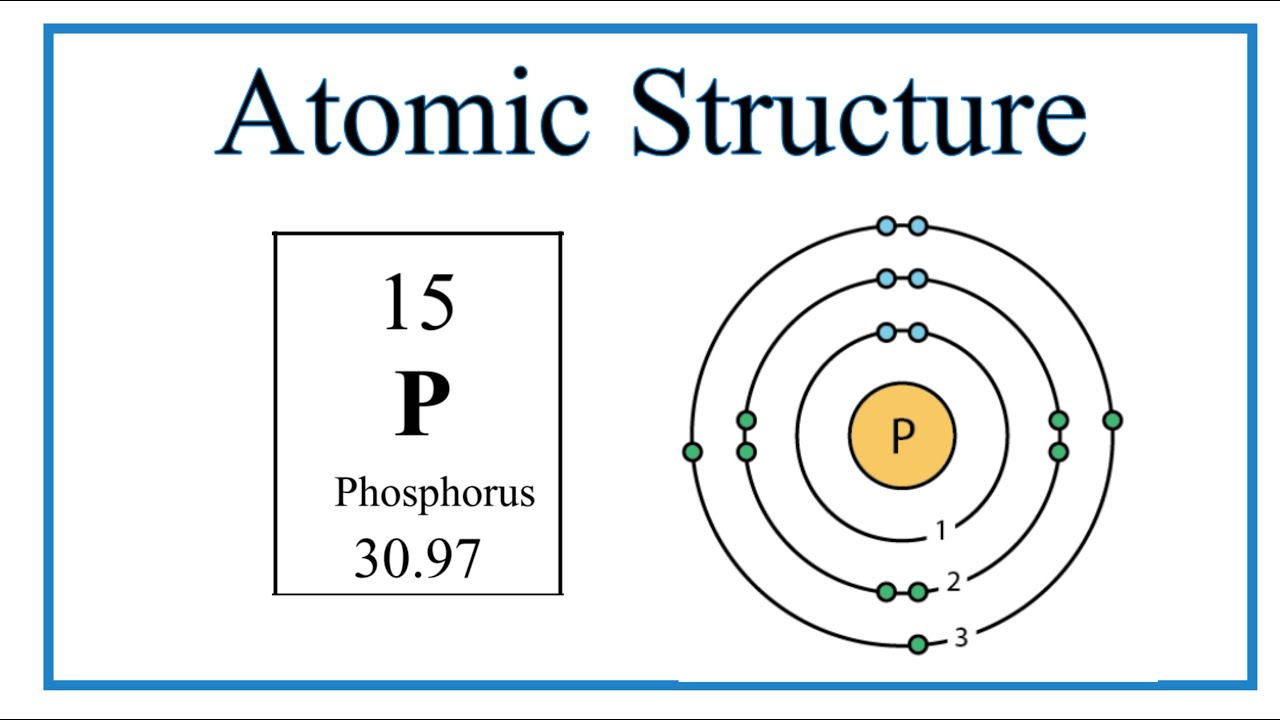
Atomic Structure (Bohr Model) for Phosphorus (P) YouTube
Figure \(\PageIndex{1}\): A Bhor's model can be used to diagram the location of electrons in each energy shell for an atom. Notice that protons go in the nucleus of the atom and electrons are drawn on orbits surrounding the nucleus. Image from Wikimedia commons. Example \(\PageIndex{2}\) Draw the Bohr's model for sodium (Na).
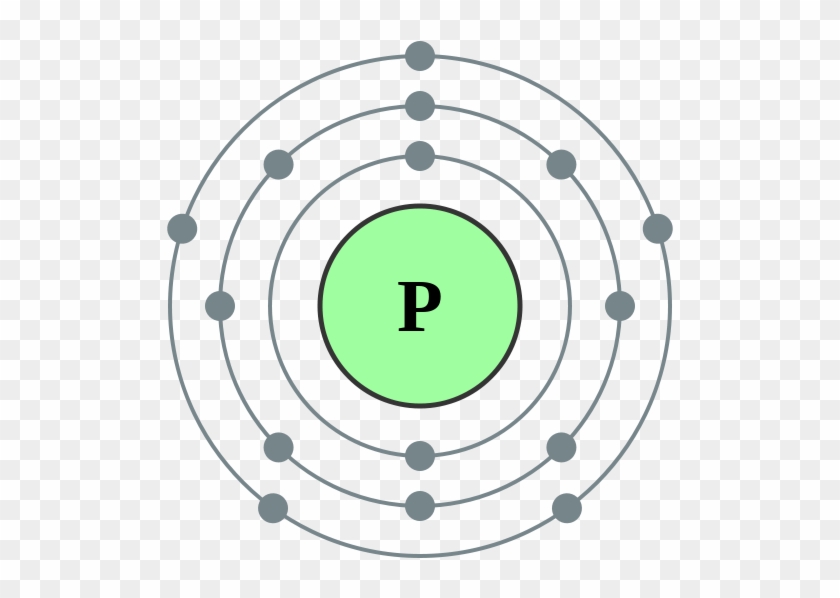
Bohr Model Of A Phosphorus Atom Electron Configuration Of Sodium
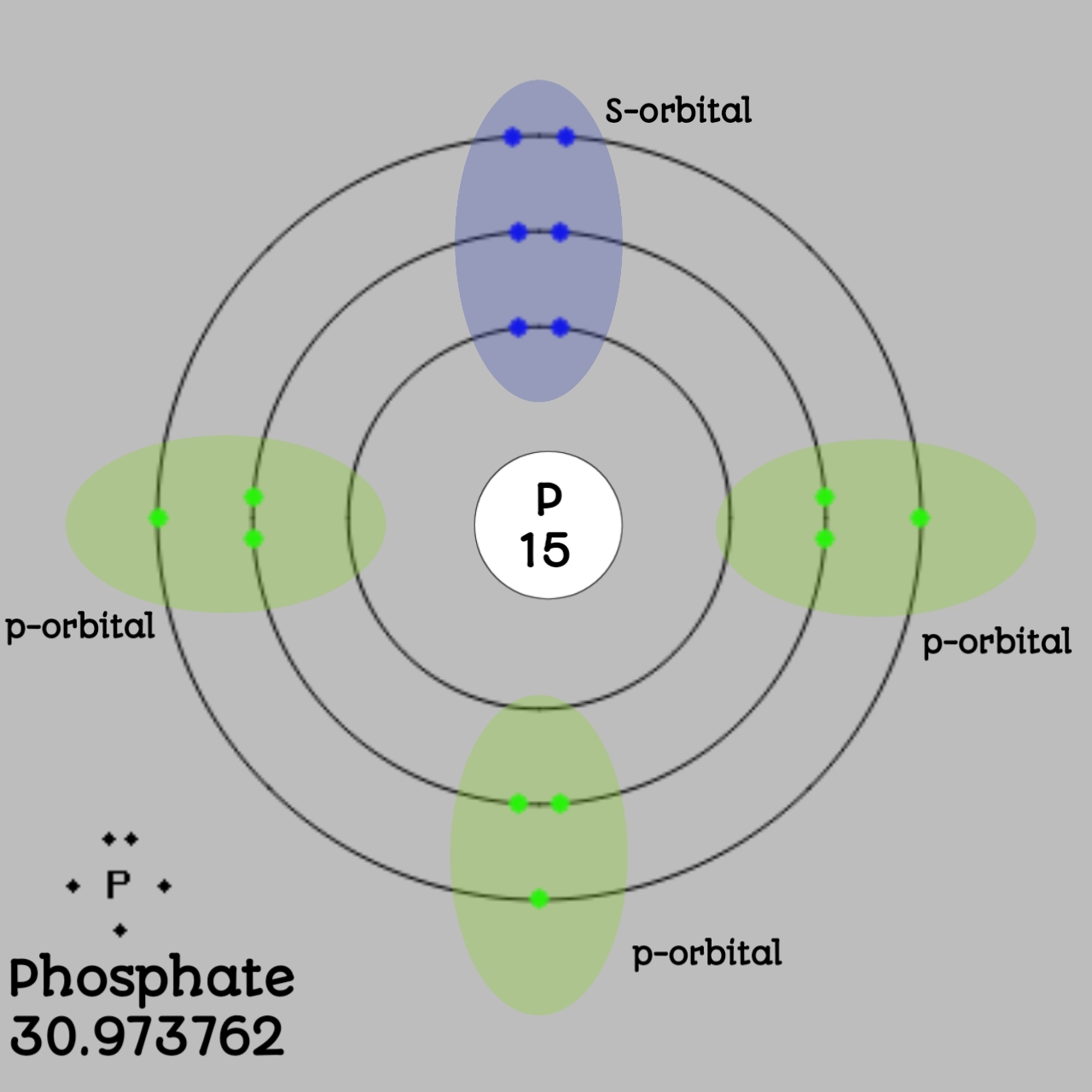
The electrons occur in three orbits. Two electrons are in Orbit 1, eight electrons are in Orbit 2 and five electrons are in Orbit 3. The outer orbit (i.e., valence) is not full. Thus, phosphorus will react with other elements to reach the maximum. Example 2: Fluorine This is the Bohr -Rutherford Diagram for Fluorine (Atomic Number 9).
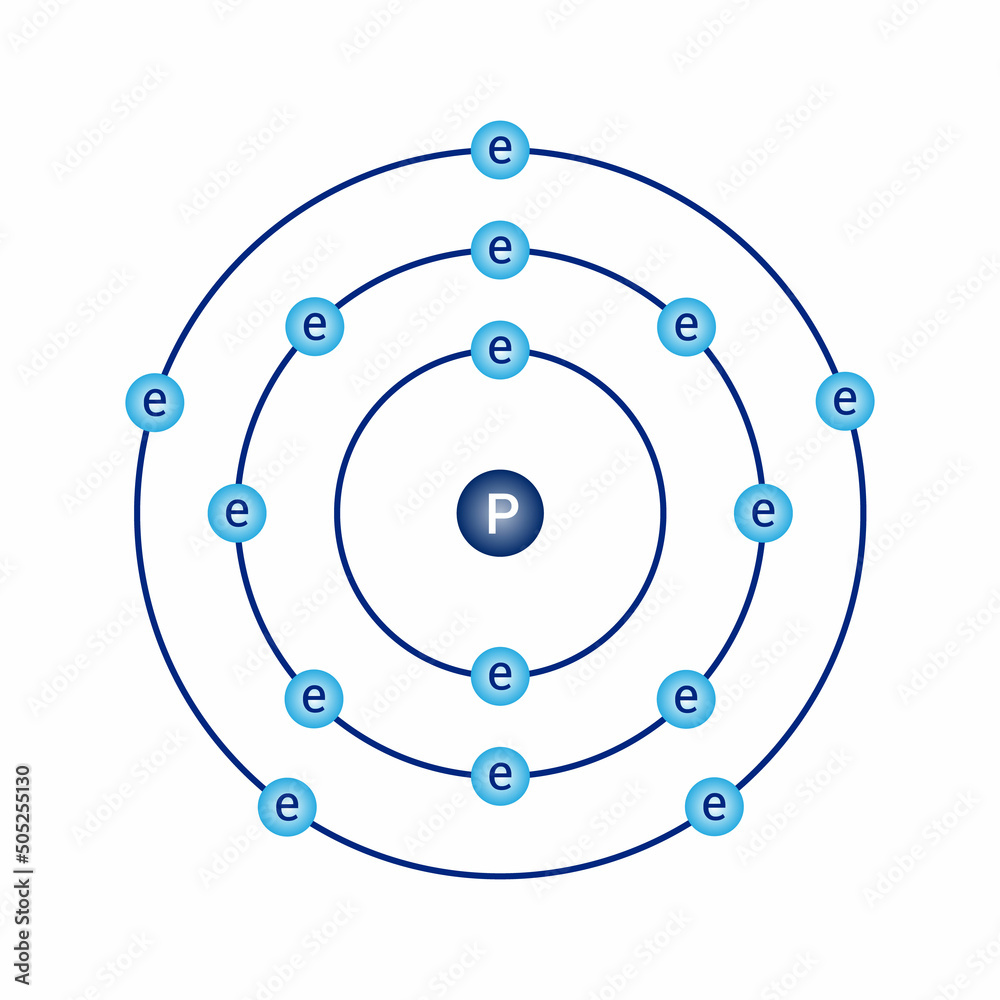
Bohr model diagram of phosphorus P in atomic physics vector de Stock
Argon has 2 electrons in its first shell, 8 in its second, 8 in its third.Check me out: http://www.chemistnate.com
Phosphorus Bohr Diagram
The Bohr model of the hydrogen atom (Z = 1) or a hydrogen-like ion (Z > 1), where the negatively charged electron confined to an atomic shell encircles a small, positively charged atomic nucleus and where an electron jumps between orbits, is accompanied by an emitted or absorbed amount of electromagnetic energy (hν). The orbits in which the electron may travel are shown as grey circles; their.

Number Of Valence Electrons In Phosphorus
The bohr Rutherford diagram for oxygen has 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second. The bohr Rutherford diagram for oxygen has 8 protons and 8.
Diagrama De Bohr Tabla
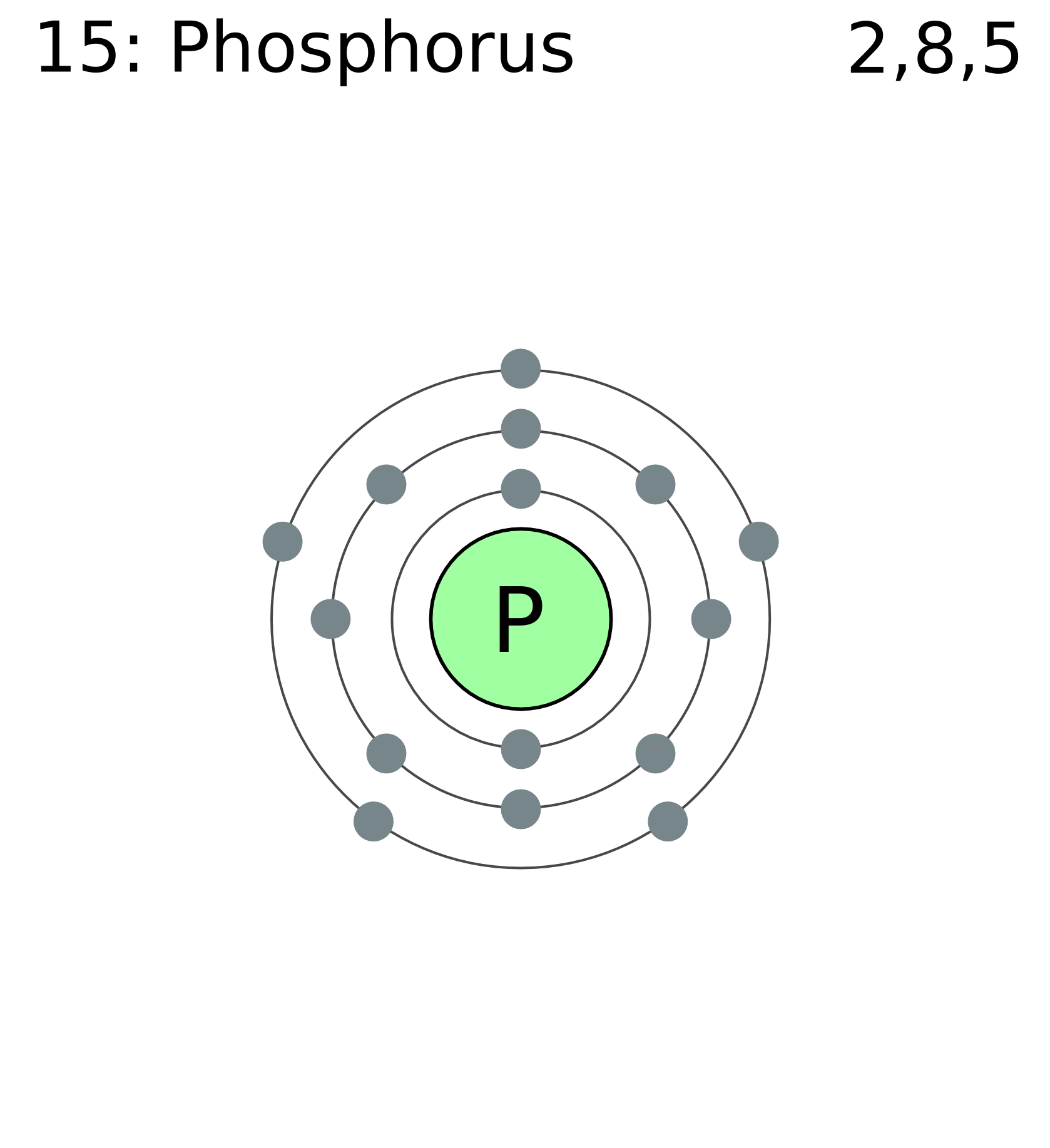
The Bohr Model of Phosphorus(P) has a nucleus that contains 16 neutrons and 15 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Phosphorus contains 5 electrons that also called valence electrons.
Phosphorus Electron Configuration (P) with Orbital Diagram
Niel Bohr, in 1913, presented the Rutherford model with the required corrections, and this model since forth has been recognized as the Bohr-Rutherford model of the atom.. Below is the video attached explaining how to draw a Bohr model diagram of a Phosphorus atom. Conclusion.

Pin on phosphorus
The Bohr - Rutherford Diagram also shows the total number of electrons is 15. Recall: The number of protons = number of electrons. The electrons occur in three orbits. Two electrons are in Orbit 1, eight electrons are in Orbit 2 and five electrons are in Orbit 3. The outer orbit (i.e., valence) is not full. Thus, phosphorus will react
draw your own bohr model for dickson only Tutor help Now
(a) Draw the Bohr-Rutherford diagram (without neutrons) for an atom of each of the following elements: lithium, oxygen, calcium; and phosphorus. (b) Draw the; Bohr-Rutherford diagram (without neutrons) for the ion formed by each of the elements in (a). (c) Write the chemical symbol for each ion.

Draw the structure of phosphorus atom according to Bohr’s model of atom
We explain how the Bohr atomic model works and provide example Bohr diagrams. Call Direct: 1 (866) 811-5546 Sign In Start Free Trial. the Rutherford Model. The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits.. Phosphorus (P) 15: 15: 16: Sulfur (S.

Phosphorus Bohr Model — Diagram, Steps To Draw Techiescientist
Identification of elements using Bohr-Rutherford Diagrams. Learn with flashcards, games, and more — for free.

Phosphorus Definition, Facts, Symbol, Discovery, Property, Uses
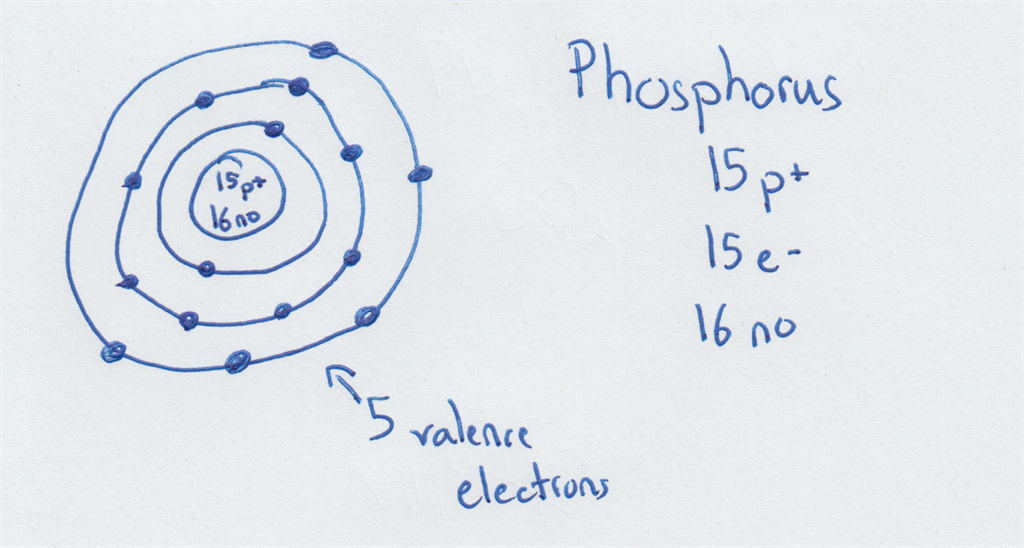
Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. The number of neutrons can be found by subtracting the number of protons from the atomic mass rounded to the nearest whole. This is because protons and neutrons.
PPT SNC2D CHEM UNIT REVIEW PowerPoint Presentation, free download
Bohr model of all Elements is mentioned in the chart below.. Phosphorus (P) 2, 8, 5: 16: Sulfur (S) 2, 8, 6: 17: Chlorine (Cl) 2, 8, 7: 18: Argon (Ar) 2, 8, 8: 19: Potassium (K) 2, 8, 8, 1: 20:. Orbital Diagram of All Elements (Diagrams given Inside) Subscribe to our newsletter. Subscription Form.

Bohr Model Example Phosphorus Diagram Quizlet
Find step-by-step Biology solutions and your answer to the following textbook question: How are the Bohr-Rutherford diagrams of nitrogen and phosphorus similar and how are they different?.